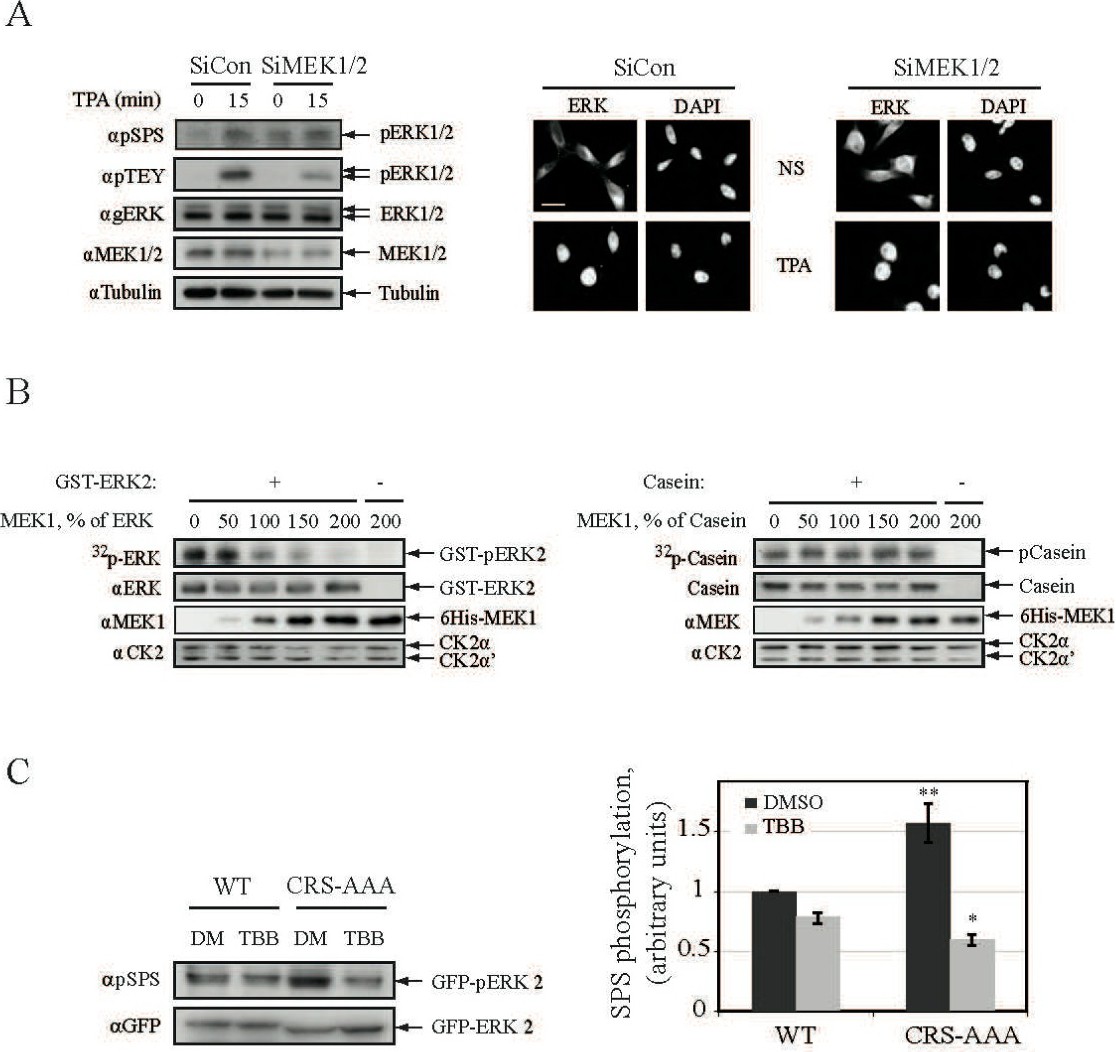
Fig. 8. The induction of CK2 phosphorylation is mediated by the release of NTS from cytoplasmic anchors. (A) Knockdown of MEK1/2 induces partial nuclear translocation and NTS phosphorylation of ERK1/2. HeLa cells were transfected with either Si-RNA of MEK1/2 or Si-RNA control and then were either stimulated with TPA (250 nM, 15 min) or left untreated as control. The cells were next harvested and their extracts were subjected to Western blot analysis using the indicated Abs. In parallel HeLa cells were grown on cover-slips followed by their transfection with Si-RNA as above. The cells were then treated as above and the nuclear translocation of their endogenous ERK1/2 was analyzed in non-stimulated cells as in Fig. 1A. Scale bar - 10 mm. (B) MEK1 prevents ERK2 phosphorylation by CK2. GST-ERK2-WT (0.1 mg per sample) was mixed in the indicated proportions with 6His-MEK1 and subjected to in-vitro CK2 phosphorylation. As a control, dephosphorylated α-Casein was mixed with 6His-MEK1 instead of ERK2. GST-ERK2 and α-Casein phosphorylation was detected by autoradiography, and the amount of the indicated proteins was detected using the appropriate Abs or by coomassie staining. (C) ERK anchoring prevents phosphorylation of its NTS by CK2 in vivo. HEK 293T cells were co-transfected with either GFP-ERK2-WT or GFP-ERK-CRS-AAA together with RFP-MEK1. After serum-starvation the cells were pretreated with TBB (10mM) or DMSO control for 2 h. Cell extracts were subjected to Western blot analysis with αpSPS-ERK and αGFP Abs. The bar-graph represents NTS phosphorylation measured by densitometry (3 experiments). The statistical differences were analyzed by student T test. (bar-graph; * P<0.01).